
How to classify high uv resistance for plastic
THE STEP EFFECT OF ultraviolet RAYS TO the Polymer RESIN as affected by ultraviolet rays will take place process description step by step: 1. Absorption ...
A. THE STEPS IMPACT OF ultraviolet RAYS TO PLASTIC
Polymer when affected by ultraviolet rays will take place process description step by step:
1. Absorbed energy wave (in which the wave ultraviolet rays have the irritation highest).
2. The process of absorption will progress to the accumulation of heat.
3. At high temperatures, the process of moving molecules and motion circuit (Brown) will reach a maximum at the zone boundary (surface) and the formation of the process, broken links, formation of free radicals (free radical).
4. Free radicals (in conditions is size analysis by thermal and vibration) will participate in the process chain reaction, causing breakdown structure polymer. During the reaction in this step, we also entice the molecular oxygen and increases activity level decomposition.
B. classifications of HIGH RESISTANCE to ultraviolet-BY-STEP THE impact OF UV
View from the steps of the process occur inside the polymer as above, one has grown out of the group, additives, to disable or confine one or several steps in the process.
According to which high resistance to ultraviolet rays are classified into several groups: absorbs UV rays (UV absorbers), lock free radicals (Free radical scarvengers), quenchers, and hydroperoxide decomposers.
- UV Absorbers (High absorption of wave energy UV)
This is the group that their molecules are sensitive to wavelengths in the ultraviolet (UV-A and UV-B), we will absorb the energy received from the wave of UV light and transfer the energy into the form of heat harmless for polymer. They act to neutralize the effect in step 1 in the process steps outlined above. Illustrations description: (1) When sunlight increases the temperature plastic material (2) When using the high absorption of UV, then reduce the phenomenon of thermal analysis, due to high UV timely radiation heat. - Quenchers
 Là nhóm hoạt chất có tính hấp thu các năng lượng nhiệt, giúp polymer phân tỏa nhiệt hạn chế quá trình tích tụ nhiệt, nhờ đó polymer không bị rung động đến mức tới hạn, nên cũng giãm quá trình mỏi và gãy mạch xảy ra. Chúng hoạt động nhằm vô hiệu hóa bước 2 trong các bước quá trình đã nêu trên. Hình trên cho thấy một số hợp chất có tính hấp thu sóng năng lượng ở mức cao (đường màu đỏ) và chuyển thành mức năng lượng ở mức thấp (đường màu đen) không ảnh hưởng đến nhựa. (Để hiểu thêm có thể đọc theo đường dẫn: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/photchem.htm)
Là nhóm hoạt chất có tính hấp thu các năng lượng nhiệt, giúp polymer phân tỏa nhiệt hạn chế quá trình tích tụ nhiệt, nhờ đó polymer không bị rung động đến mức tới hạn, nên cũng giãm quá trình mỏi và gãy mạch xảy ra. Chúng hoạt động nhằm vô hiệu hóa bước 2 trong các bước quá trình đã nêu trên. Hình trên cho thấy một số hợp chất có tính hấp thu sóng năng lượng ở mức cao (đường màu đỏ) và chuyển thành mức năng lượng ở mức thấp (đường màu đen) không ảnh hưởng đến nhựa. (Để hiểu thêm có thể đọc theo đường dẫn: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/photchem.htm)
- Free radical scavengers (Group lock the activity of free radicals).
Polymer affected by the energy factor such as: mechanics, heat, wave energy (which has wave UV, infrared, etc.) and substances that are oxidized (oxygen, peroxy,...), both occurring process fracture of the circuit, and formed the original investment do not expect. The image above shows the locking compound radicals (wave white) will promptly converts to activate the surround component is increased by heat, and promptly extinguish free radicals just when formed.
Because the group of additives are key activity of the free radicals not only help the polymer is more durable when exposed to ultraviolet rays, but also useful in the process of anti-degeneration for the polymer in the process of aging, is the mechanical impact excessive.
The quality features on and effective operation in low temperature conditions (like when the polymer is exposed to light) will be used as the high resistance to ultraviolet rays.
Group of high ultraviolet resistance of this type have a number of sub-groups is very common and important, such as HALS (Hindered Amines Light Stabilizers), Hinered Phonols, Thiosynergist,...
Activities of the group key activity of free radicals (Free radical scavengers)are intended to confine and disable the process that occurs in step (3) and (4). - Hydroperoxide decompose (Group key activity of the hydroperoxide)
In the process of the transfer of the group containing free radicals the radicals have an impact on the oxygen atom (in the environment) and the formation of hydroperoxide. The hydroperoxide is one of the factors that cause a huge impact on the degenerative process of the polymer. Group additive to immobilize and disable the hydroperoxide is the high resistance to ultraviolet rays, effectively, we have disabled the process that occurs in step (4) as mentioned above. The majority of UV are heading lock active free radicals also features lock the formation of free radicals due to the hydroperoxide decomposition. But we can also use a number of compounds are disinfectants to remove a proactive, the hydroperoxide n









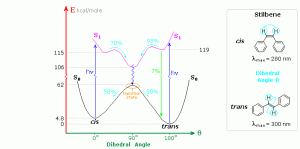 Là nhóm hoạt chất có tính hấp thu các năng lượng nhiệt, giúp polymer phân tỏa nhiệt hạn chế quá trình tích tụ nhiệt, nhờ đó polymer không bị rung động đến mức tới hạn, nên cũng giãm quá trình mỏi và gãy mạch xảy ra. Chúng hoạt động nhằm vô hiệu hóa bước 2 trong các bước quá trình đã nêu trên. Hình trên cho thấy một số hợp chất có tính hấp thu sóng năng lượng ở mức cao (đường màu đỏ) và chuyển thành mức năng lượng ở mức thấp (đường màu đen) không ảnh hưởng đến nhựa. (Để hiểu thêm có thể đọc theo đường dẫn: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/photchem.htm)
Là nhóm hoạt chất có tính hấp thu các năng lượng nhiệt, giúp polymer phân tỏa nhiệt hạn chế quá trình tích tụ nhiệt, nhờ đó polymer không bị rung động đến mức tới hạn, nên cũng giãm quá trình mỏi và gãy mạch xảy ra. Chúng hoạt động nhằm vô hiệu hóa bước 2 trong các bước quá trình đã nêu trên. Hình trên cho thấy một số hợp chất có tính hấp thu sóng năng lượng ở mức cao (đường màu đỏ) và chuyển thành mức năng lượng ở mức thấp (đường màu đen) không ảnh hưởng đến nhựa. (Để hiểu thêm có thể đọc theo đường dẫn: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/photchem.htm)